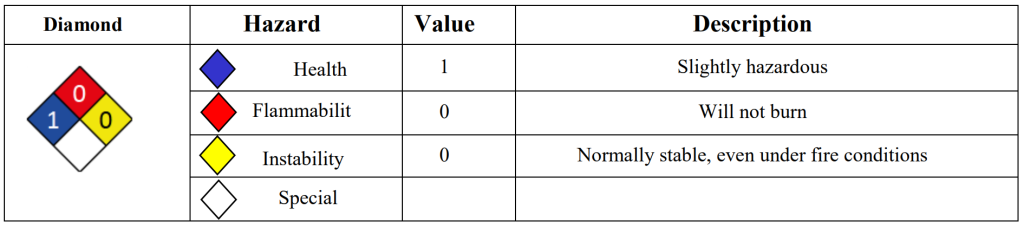
| Flammability limit | Threshold limit value | ||
|---|---|---|---|
| LFL | UFL | TLV-TWA | TLV-STEL |
| 3.2% | 15.3% | 25 ppm | 10 mg/m3 |
Anti freeze: General Description And Use
:DESCRIPTION
Antifreeze is an additive that lowers the freezing point of a water-based liquid. An antifreeze mixture is used to achieve freezing point depression in cold environments. Common antifreezes also increase the boiling point of the liquid. Early engine coolant antifreeze was methanol (methyl alcohol). Ethylene glycol was developed because its higher boiling point was more compatible with heating systems. Most vehicle and engine coolant manufacturers recommend a 50% (vol) concentration be used for year round use. This concentration is needed to achieve the engine design cooling capacity, to provide a margin of safety against boilover, to ensure an adequate level of inhibitor concentration and to provide freeze protection to —34°F (—37°C). An important feature of today’s increasingly used coolant is its higher boiling point. Now a days the higher boiling point of glycol engine coolant makes it possible for the automobile engine to operate at higher temperatures.
:USE
- Prevent freezing
- Prevent boiling
- Descaling
- Protect metal parts
GHS hazard statements
H302 – Harmful if swallowed
H373 – May cause damage to organs (kidneys) through prolonged or repeated exposure (on ingestion)
GHS precautionary statements
P101 – If medical advice is needed, have product container or label at hand
P270 – Do not eat, drink or smoke when using this product
P501 – Dispose of the containers in accordance with local/regional/national/international regulations.
P301+P312 – IF SWALLOWED: Call a doctor, a POISON CENTER if you feel unwell.